Psychedelics are a class of psychoactive substances that produce changes in perception, mood and cognitive processes. Also so known as “hallucinogens”, reference to psychedelics is commonly meant to include substances such as Psilocybin (a.k.a. “magic mushroom”), MDMA (a.k.a. “extasy”) and LSD (Lysergic acid diethylamide).
Likely following the lead of the ongoing legalisation of cannabis in Canada, many U.S. states and other countries, the recent years have seen unprecedented investments and an increasing interest in research, production, and supply of psychedelics, particularly for potential medicinal uses.
The present article aims to provide a brief overview of the current situation from a patent perspective and to discuss about patenting strategies for these substances.
Patent filing activity
A very simple keyword search in patent databases using the terms “hallucinogenic” or “psychedelic” in either the Title, Abstract or Claims revealed a total a 189 patents (81 families) filed since 2011[1]. As shown in Figure 1 below, yearly patent filings are increasing, showing an exponential growth in the last three years.
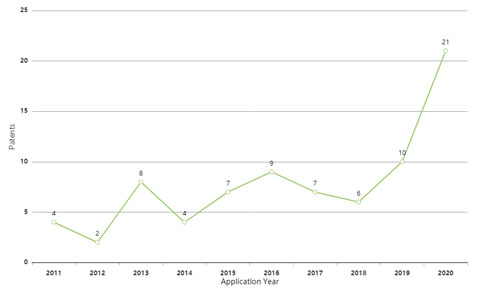
Figure 1: Yearly patent filing for psychedelics
The subject matter covered by these issued patents or pending patent applications is broad and include new medical uses such as treatment of headaches, treatment of CNS disorders, depression, and food allergies to name a few. Patents documents also cover new compounds and derivatives, methods and vehicles for delivery, etc. Table 1 below provides a few recent examples of patent documents in this field.
Table 1: Recent patent documents relating to psychedelics
| Applicant | Patent or patent application number | Title |
| MINDSET PHARMA INC. . | WO 2021/155470 | Psilocin derivatives as serotonergic psychedelic agents for the treatment of CNS disorders |
| WO 2021/155467 | 3-pyrrolidine-indole derivatives as serotonergic psychedelic agents for the treatment of CNS disorders | |
| YALE UNIVERSITY | US 2021/0236523 | Psychedelic treatment for headache disorders |
| CONCEPT MATRIX SOLUTIONS | US 2021/0015833 | Oral soft gel capsule containing psychedelic compound |
| SW HOLDINGS, INC. | WO 2021/003467 | Metered dosing compositions and methods of use of psychedelic compounds |
| APOTHECA SYSTEMS INC. | WO 2020/237394 | Method and system for personalizing and standardizing cannabis, psychedelic and bioactive products |
| COMPASS PATHFINDER LIMITED | WO 2020/212952 | Treatment of depression and other various disorders with psilocybin |
| GH RESEARCH LIMITED | WO 2020/169851 | Compositions comprising 5-methoxy-n,n-dimethyltryptamine (5-meo-dmt) for use in treating mental disorders |
| PALO ALTO INVESTORS LP | US 11,045,454 | Methods of treating food allergy conditions |
| ELEUSIS HEALTH SOLUTIONS US, INC. | US 2021/0183519 | Methods and systems for enhancing safety of psychedelic drug therapies |
Patenting strategies
For being patentable, an invention must be “new” and “inventive”. Since most common psychedelics are known for many years, it is not possible to patent these existing substances (i.e. lack of novelty).
Although the psychedelics in their natural form cannot be patented, there is still room for patent protection in this field. For instance, new medical uses of the existing compounds can be patented as well as new derivatives of the natural molecules. Additional patentable subject matter may also include novel delivery methods, novel formulations, novel synergistic combinations active ingredients, and novel dosage regimens. It may also be possible to patent the uses of the psychedelics within particular patient populations (e.g. genetic trait or biomarker).
Considering the patenting challenges, it will be very important for psychedelic developers to make sure they have sound strategies around intellectual property. Absence of intellectual property will most certainly make the company or project not so interesting for investors or commercial partners.
Conclusions
The interest in psychedelics is increasing and it looks like this field is poised to grow, following the path of cannabis legalization in Canada, many U.S. states and other jurisdictions. There is a rapidly increasing number of patents being filed for psychedelics and companies that are serious should develop early intellectual property strategies for protecting their innovations and also for making sure not to infringe someone else patents.
[1] Search carried out early September 2021. The search is not exhaustive since many relevant psychedelics-related patents likely do not comprise the particular keywords used. For instance, psychedelic parents may recite instead keywords such as” psilocybin”, “MDMA”, “LSD”, etc.
Learn more about our cannabis practice and our trademark practice.
Serge Lapointe has extensive experience in patent protection in the life sciences sector in Canada, the United States and abroad. Serge combines his vast knowledge and his experience when drafting patent applications and preparing opinions on the validity and infringement of biotechnology, pharmaceutical and chemical patents. He gets often involved into intellectual property due diligence investigations.
Serge has more than 20 years of experience working in firms and in industry, and he has acquired in-depth knowledge of the scientific and regulatory aspects of drug development. His ability to examine technology from a pragmatic business perspective is an added value for clients.
Serge responds effectively to the legal and commercial needs of a varied clientele from the genomics, proteomics, pharmaceutical products and chemical industries.
He has considerable experience in assisted reproductive technologies, diseases of the central nervous system, obesity, diabetes, virology, microbiology, genetic markers, blood and nephrological disorders, stem cells, nutraceuticals, and green technologies.


